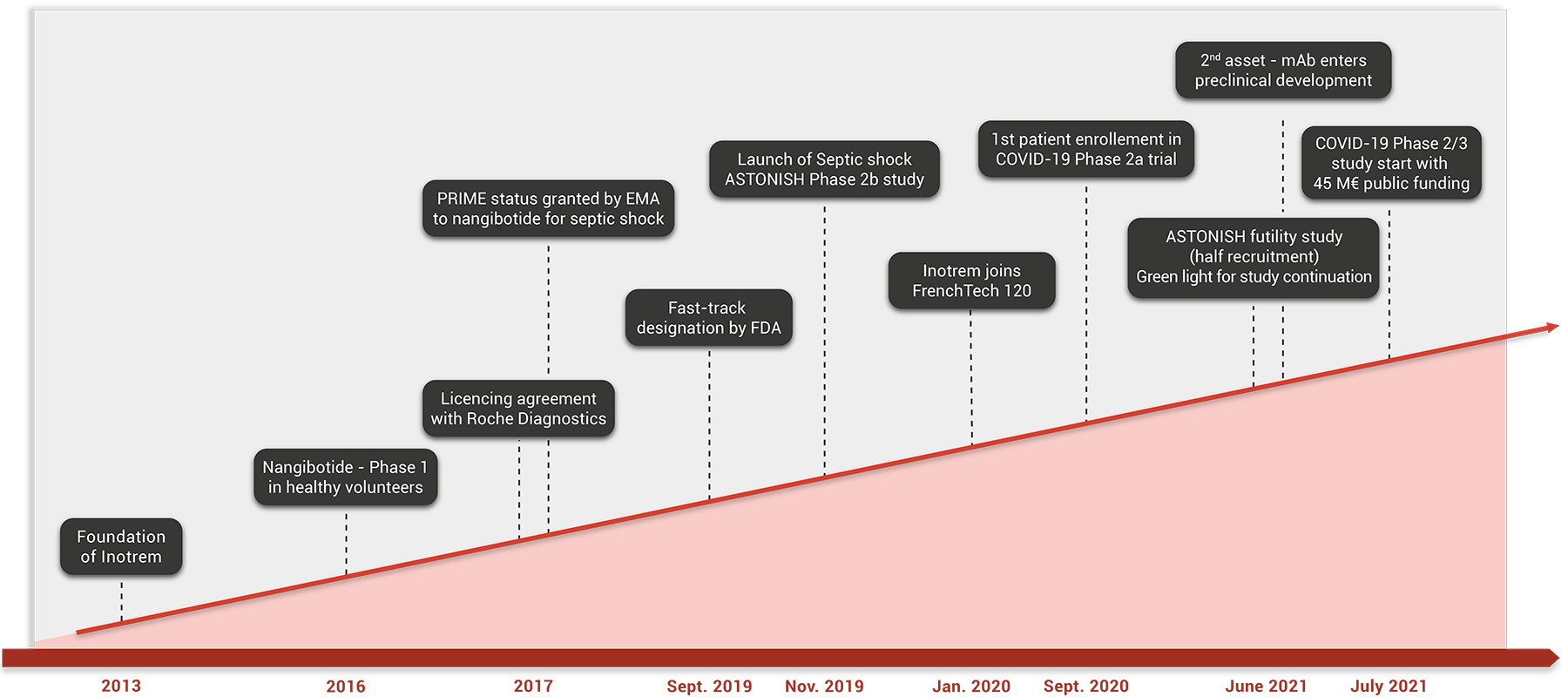
A novel approach to target inflammatory diseases
Inotrem is an advanced clinical stage biotech company specialized in immunotherapy to control dysregulated inflammatory reactions.
The company proposes a new concept of controlling innate immunity that targets the TREM-1 pathway, an innate immune amplifier, to restore a balanced response in inflammatory diseases.
Inotrem is developing nangibotide, a peptide and first-in-class TREM-1 inhibitor, with applications in severe conditions such as septic shock and COVID-19. Leveraging its proprietary technology platform, Inotrem is also conducting a broad monoclonal antibody program to target chronic inflammation, fibrotic diseases and immuno-oncology.
Inotrem’s deep knowledge of the TREM-1 pathway biology and role in inflammation allows to develop in parallel companion diagnostics tools to select those patients more likely to benefit from the anti-TREM strategies.