The efficacy was demonstrated in the phase 2b ASTONISH trial (NCT04055909)(4). An optimal prognostic cut-off for baseline plasma sTREM-1 allows to identify the most severe patients that do not respond to standard of care and are at high risk of mortality (5). This optimal cut-off allows to discriminate 2 patient populations: patients above the prognostic cut-off are at high risk of morbidity and mortality, and patients below this cut-off show much lower severity. Plasma sTREM-1 thus identifies patients in shock at high risk of death and whose organ dysfunctions are heavily mediated by TREM-1.
Nangibotide effect is only observed in high sTREM-1 patients: these patients do not respond to standard of care, and a significant improvement in organ function was observed when nangibotide was administered on top of standard of care (measured by a time-dependent decrease in SOFA score (Sequential Organ Failure Assessment score), see adjacent figure, top panel). Patients with lower levels of sTREM-1 respond well to standard of care, and no added value of nangibotide was observed in this subgroup (Figure, bottom panel).
The proportion of high sTREM-1 patients represents approximately 45% of all septic shock patients.
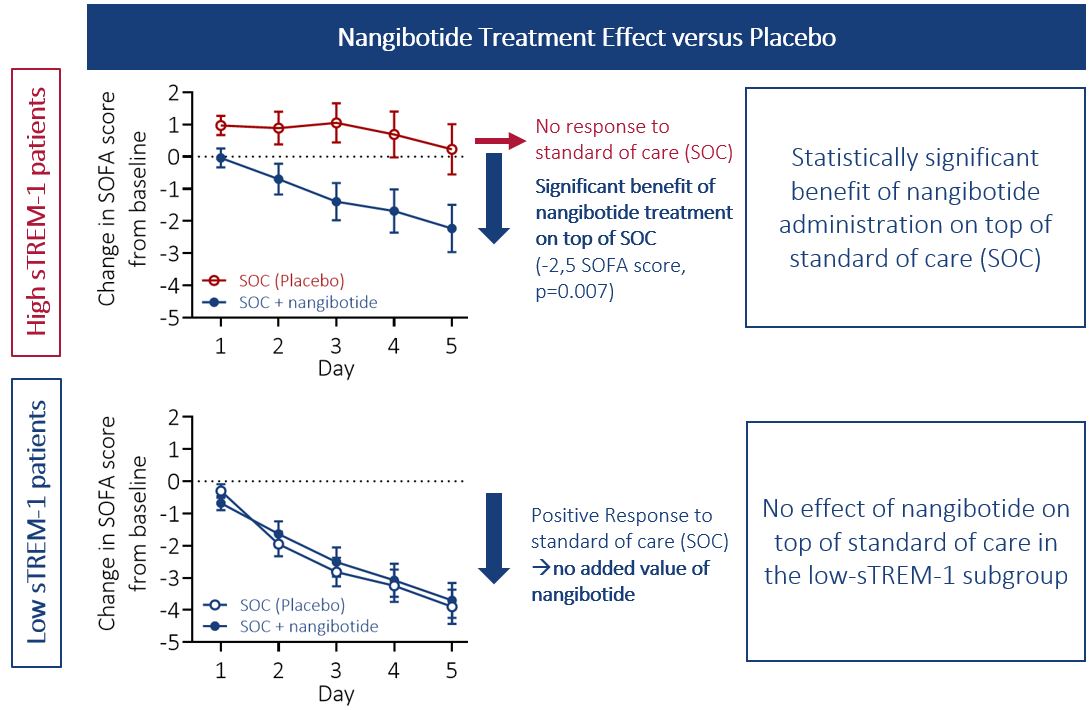
In high sTREM-1 patients, the effect of nangibotide restoring organ function translated into a reduction in shock duration, length of stay in the ICU, as well as of long-term morbidity and mortality. Indeed, at day 28, mortality was reduced by 22%, and the proportion of patients being alive and free of organ support was improved by 32%. This effect was still present at day 90 (-25% mortality relative to placebo).
-2.5 points SOFA score at day 5
versus placebo following treatment initiation
p=0.007
+42% shock reversal within 5 days
versus placebo
p=0.006
+32% patients Alive and Free of Organ Support at day 28
relative to placebo
p=0.119
-25% mortality at Day 90
relative to placebo
p=0.141
-2.9 days length of stay in the ICU
p=0.100
+3.6 days organ support free days
p=0.082