Developing innovative immunomodulatory therapies targeting the TREM-1 pathway to restore balanced inflammation in life-threatening inflammatory conditions
Specific inhibitors of the TREM-1 pathway, an innate immune amplifier leading to inflammation overdrive. Extensive preclinical studies demonstrate efficacy across multiple inflammatory disease models.
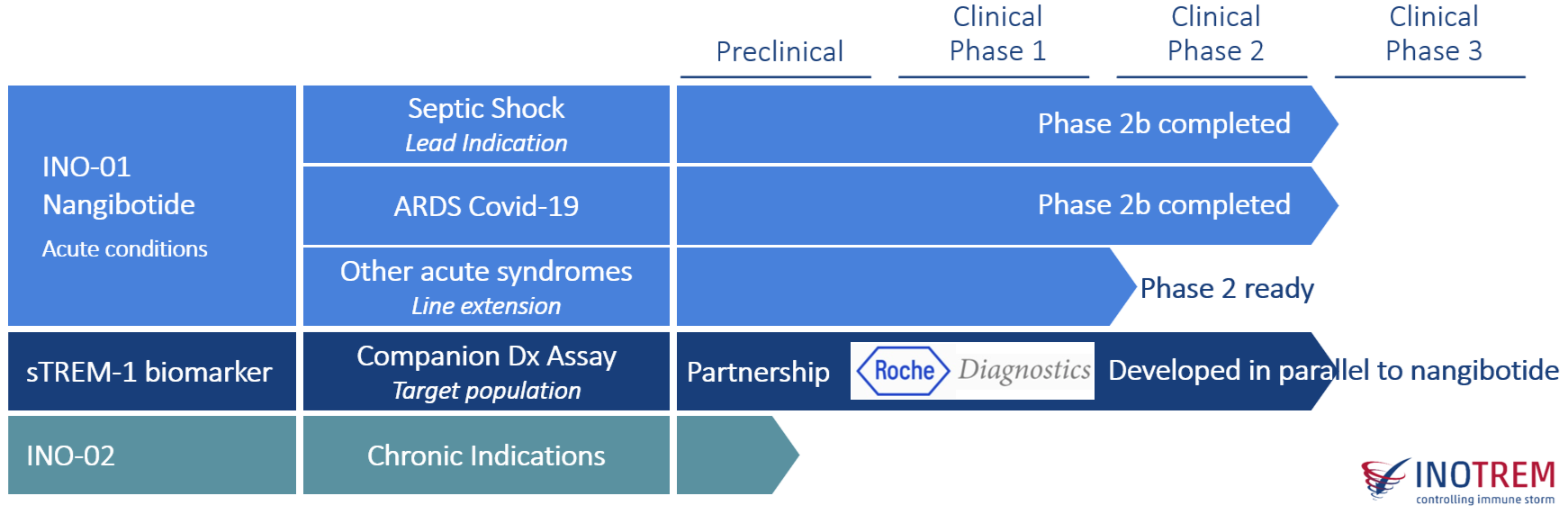
Our lead candidate INO-01 / nangibotide has shown robust clinical proof of safety and efficacy in Phase II clinical trials for septic shock and severe COVID-19.
We developed with Roche Diagnostics a biomarker platform to identify the target patient population for our therapies.
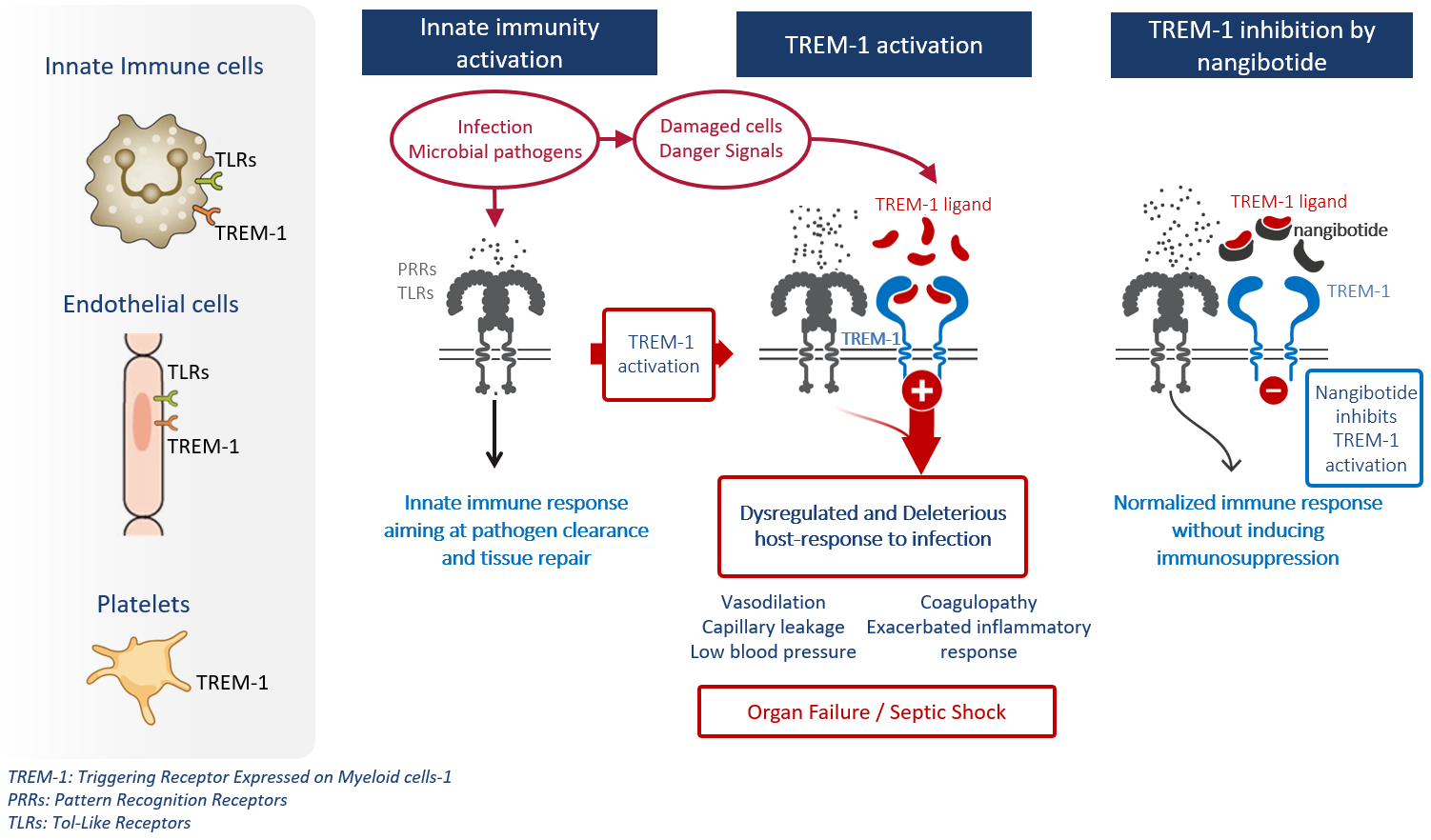
TREM-1 has been identified as a key disease driver in acute and life-threatening inflammatory diseases, where it is expressed on innate immune cells such as monocytes/macrophages, neutrophils, endothelial cells, and platelets. In these cell types, it is upstream of multiple known inflammatory pathways and acts as an amplifier of inflammation.
Indeed, TREM-1 triggers inflammatory signals through a functional crosstalk with the Pattern Recognition Receptors (PRRs), including Toll-Like Receptors (TLRs), which are the first line of host defense against microbial pathogens by recognizing pathogen and danger-associated molecular patterns (Figure below).
When TREM-1 is co-activated with these PRRs, an exuberant and dysregulated inflammatory response develops: TREM-1 amplifies the signals mediated by PRRs, contributing to the establishment of a deleterious inflammatory response.
As such, in septic shock, TREM-1 promotes hyperactivation of the innate immune response and endothelial cells inflammation, leading to cytokine storm, vascular leakage, and disseminated coagulation, and ultimately organ failure (1, 2). TREM-1 is known today to be a central mediator in various acute and chronic inflammatory diseases.
TREM-1 receptor activation is also characterized by the release of a soluble form of the TREM-1 protein (sTREM-1), which is a marker of the activation of the pathway (3). Plasma sTREM-1 level thus reflects the contribution of the TREM-1 pathway in the pathophysiology of diseases. Notably in septic shock, sTREM-1 is a biomarker allowing to identify severe patients with a high level of TREM-1 activation leading to TREM-1 mediated organ dysfunctions and who are likely to respond to nangibotide. This concept was fully validated in our phase 2b ASTONISH clinical trial.
INO-01 / Nangibotide, Inotrem's most advanced asset, is a first-in-class peptidic TREM-1 inhibitor initially developed to address a major unmet medical need: septic shock.
Nangibotide is a host-directed, pathogen agnostic therapeutic solution developed in combination with the mechanism-based biomarker sTREM-1, the soluble form of TREM-1 measured with an immunoassay developed with Roche Diagnostics. The sTREM-1 assay identifies patients with TREM-1 mediated organ dysfunctions, thereby introducing a breakthrough innovation: the first precision medicine approach in acute care.
Nangibotide is a specific inhibitor of TREM-1, and as such, it blocks the amplification loop of the innate immune response mediated by TREM-1, and ultimately restores balance and control in the inflammatory response without immunosuppression. In septic shock, this led to an improvement of organ function in the early phase of septic shock, which translated into mid and long-term benefit.
Nangibotide completed a phase 2b trial in 355 septic shock patients as well as a phase 2b clinical trial in 220 severe COVID-19 patients. Nangibotide has been labelled PRIME (PRIority Medicine) by the European Medicine Agency (EMA) and has received the Fast Track designation by the U.S. Food and Drug Administration (FDA). The companion diagnostic assay has received the Breakthrough Device Designation (BDD) by the FDA. Regulatory path up to registration has been cleared with the FDA, EMA, and PMDA.
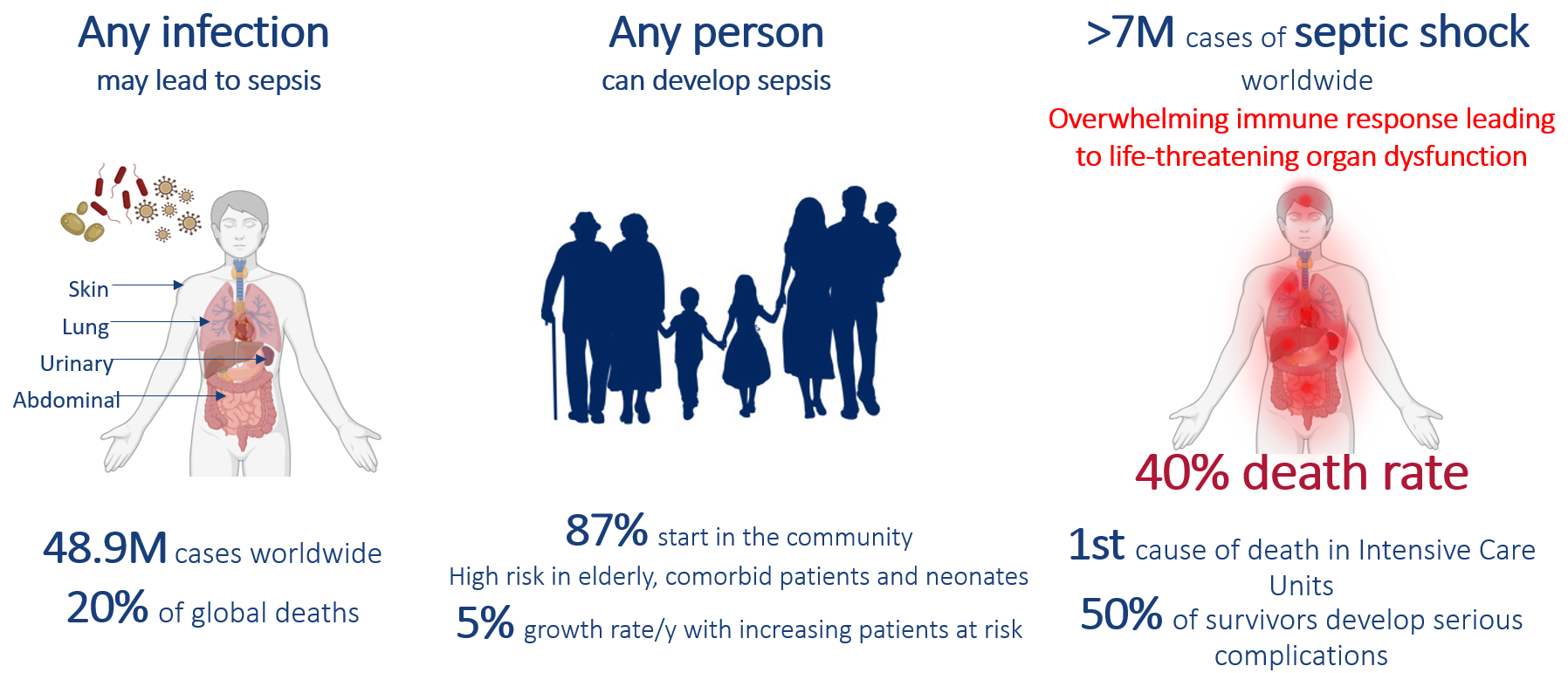
Septic shock is characterized by a dysregulated immune response to infection, leading to organ failure and high mortality. It is an overwhelming immune response leading to life-threatening organ dysfunction: the condition reflects how the body's own defense can become deadly.
Each year, septic shock affects over 700,000 people in Europe, causing up to 250,000 deaths. Septic shock is the leading cause of Intensive Care Unit (ICU) mortality and contributes to 1 in every 3 deaths in the hospital. It imposes major healthcare burden, with many survivors facing long-term complications and reduced quality of life.
No specific treatment exists! Current therapies only manage symptoms, not the underlying immune dysfunction. There is a urgent need for biomarker-guided, targeted therapies to improve outcomes.
=> Learn more about sepsis and septic shock: sepsis.org ; World Health Organization ; Global Sepsis Alliance ; The end of the one-size-fits-all
Inotrem built a robust development program based on 4 main pillars with the mission to succeed with nangibotide. We aim at providing the first causal treatment for patients suffering from life-threatening acute inflammatory syndromes due to dysregulated immune response driven by TREM-1. Septic shock being the first indication.
that reflect our cutting-edge understanding of the pathophysiology of septic shock.
TREM-1 is an immune overdrive and is upstream of most known inflammatory pathways. Inhibiting TREM-1 allows to restore control in the inflammatory response without immunosuppression.
Use of a mechanistic biomarker to target patients populations.
sTREM-1 is a mechanism-based biomarker that reflects TREM-1 activation.
sTREM-1 identifies severe patients who suffer from TREM-1 mediated organ dysfunction, do not respond to standard of care, and are likely to respond to nangibotide.
Essential for late-stage trials.
have reduced patient heterogeneity, improving septic shock trial reliability and reproducibility.
as it requires enrolling a very large number of patients.
based on a new composite endpoint that captures both mortality and morbidity, reducing required patient numbers and reflecting broader impacts like long-term morbidity.
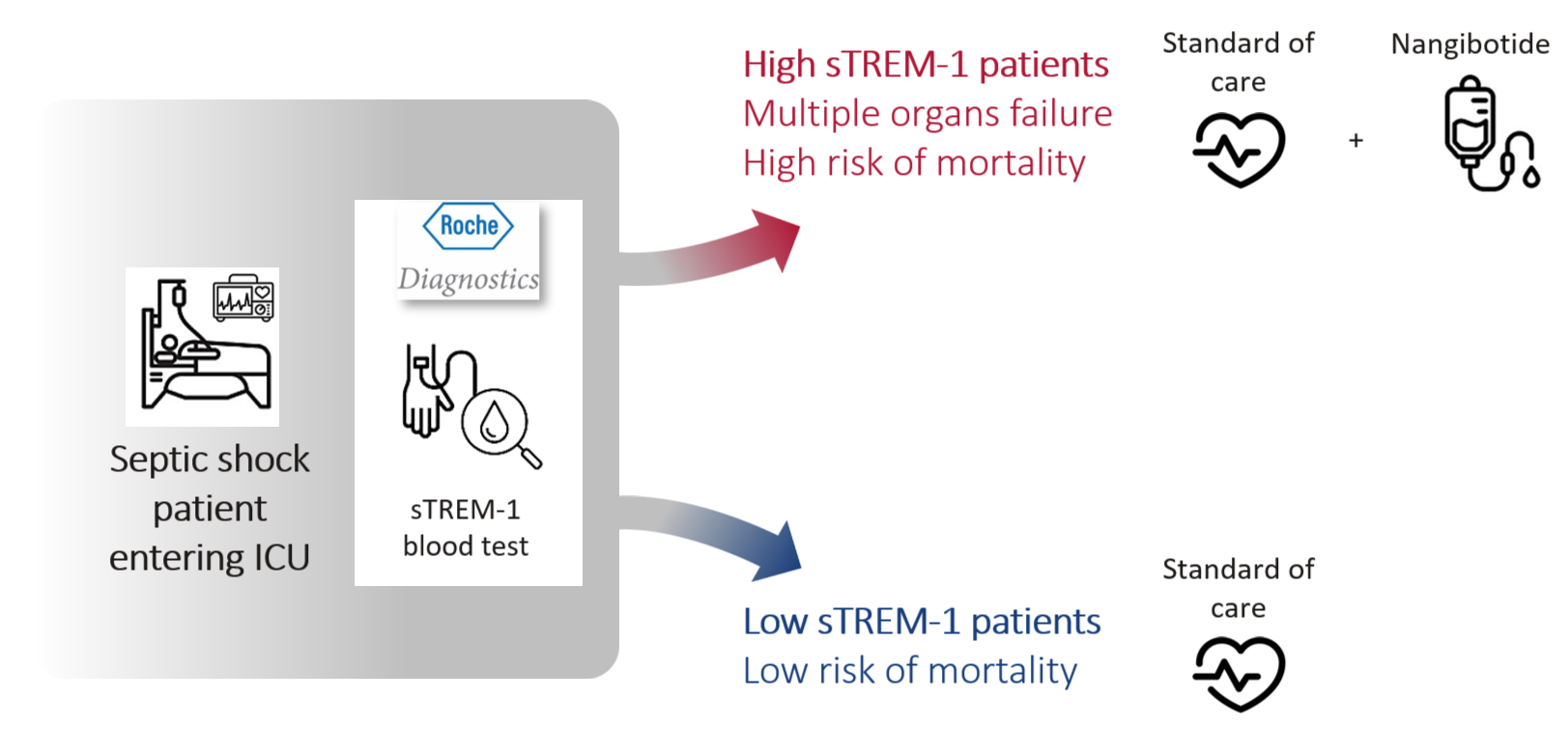
Inotrem aims at introducing the first precision medicine approach in intensive care with nangibotide, an inhibitor of TREM-1 activation, coupled with the use of soluble TREM-1 (sTREM-1), the marker of TREM-1 activation, to identify patients in shock at high risk of death and whose organ dysfunctions are heavily mediated by TREM-1.
After diagnosis of septic shock and transfer to ICU, blood sTREM-1 levels will be measured using a close to care diagnostic test (Roche Diagnostics). sTREM-1 measurement will guide the decision whether or not to administer nangibotide treatment: if the sTREM-1 level is above the defined cut-off value (“high sTREM-1 patients”), the patient is declared eligible and treatment with nangibotide is initiated as a continuous intravenous infusion on top of standard of care and maintained for a duration of 5 days.
The company reports statistically significant improvement of organ function in septic shock patients with high-sTREM-1 levels. Read our research letter in Intensive Care Medicine journal.
Read MoreInotrem announces agreement with the FDA for a Phase 3 registration trial for nangibotide in septic shock
Read MoreInotrem announces publication of two key articles on nangibotide Phase II programs in the prestigious peer-reviewed journals The Lancet - Respiratory Medicine and The Lancet - eBioMedicines.
Read More
Chief Executive Officer
Broad experience in strategic development of advanced clinical stage biotech companies

Cofounder and Executive Vice-President, Head of Scientific and Medical Affairs
30+ years in pharmaceutical R&D leadership with expertise in translational medicine.

Chief Operating Officer
Senior executive with international experience in finance and operational functions.

Chief Development Officer
Senior executive with broad international experience in drug development.

Chief Technical Officer and Head of Manufacturing
Senior executive with deep experience in development process of chemical and protein entities
Interested in learning more about our work or potential collaborations? We'd love to hear from you.
9 Avenue de la Forêt de Haye
54500 Vandoeuvre-les-Nancy, France